Interventi
Descriptionof miniliposuction technique.
1) Consultation and signature in the informed consent, forty minutes, sometimes more.
2) Miniliposuction: fifteen minutes. This is the technique.
Perform the miniliposuction after disinfection of the area of skin (abdomen or hips, the abdomen has a better fat) where fat should be harvested: the harvesting area in as large as a hand. Administration of 50 cc of saline solution with anesthetic and epinephrine (called solution of Klein and well known and used all around the world) with a normal syringe needle, or better a Klein cannula, through a small incision of 1.2mm.
Then, a particular 1,2mm needle (a Coleman cannula) connected to a 30 cc syringe can run the aspiration of fat with the power of aspiration exerted only by the operator's hand on the plunger of the syringe (no need for a vacuum device, rather it is contraindicated because fat must be treated gently and not managed under excessive pressure).
Obtained 30 cc (or less) of fat, then the closure of the little incision with a small plaster (steristrip) or a stitch is performed. Apply antibiotic ointment. Patch. Prescription of oral antibiotics to take at home.
The only contraindication is the use of anticoagulants (eg Coumadin for patients in atrial fibrillation or history of pulmonary thromboembolism that are contraindications by themselves).
Attention must be paid for avoiding allergic reaction to disinfectant during skin disinfection at the beginning of the procedure and to antibiotic ointment put on skin under the dressing at the end of it , or to the antibiotic pills prescribed for use at home.
Obvious exclusion if there has been any previous infectious disease.
The fat is distributed with great care and delicacy in three vials of sterile plastic, together with 8 mg of gentamicin and a few cc of saline, and placed in a polystyrene box for shipping by international courier.
The female patient's husband has authorized me to release the data that I expose
The report was written by the female patient's husband himself and is only minimally "adapted" by me.
Clinical summary of what happened
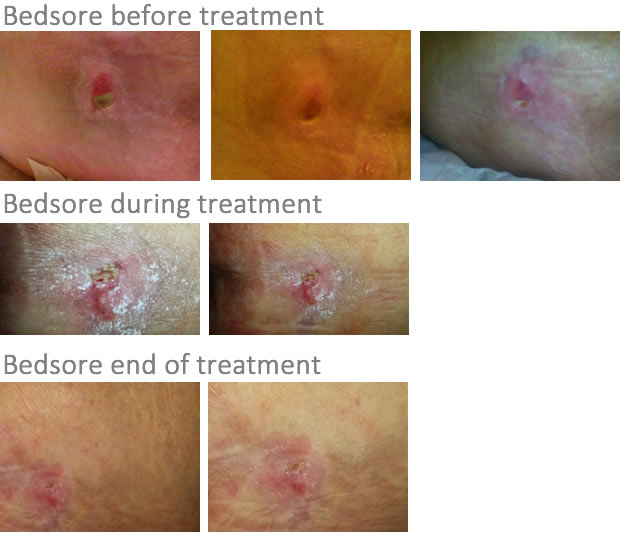
Following an admission to an intensive care ward, in January 2014, (the name of the Hospital is not indicated) the patient A.A. , a woman suffering from ALS and definitely lying in bed, did not receive any mobilization. This resulted in the formation of a pressure ulcer (a bedsore) in the sacrum area. All this was discovered after about 10 days in another hospital (note: where the patient was then transferred, after discharge from the ICU where she had never been mobilized).
Since then, she began to be treated daily with standard medical ointments as IRUXOL (collagenase plus chloramphenicol) and FITOSTIMOLINE (triticum vulgare plus phenossyethanol) cream.
After about 3 months of standard treatment, the bedsore was visibly worsened.
Once at home, after having got some opinion from family doctor and some nurses and others, each one with contrasting ideas, I would achieve (note: it is the husband who speaks) an opinion from a surgeon, who suggested a surgical cleaning to be done monthly.
Unfortunately the surgical debridment was not done because the surgeon (who had been working in an hospital facility and he was not on private practice) was not allowed to come at our home.
In the meantime the plague had been worsening more and more. From March 2014 to August 2014, three cleaning procedures of debridment had been performed in a private facility. At first a slight improvement seemed to be brought about, but after a few days the situation worsened again , and returned to a condition where, indeed, the underlying bone could be seen (note: these are her husband's words).
In September 2014 , through Mr. Peter Kellner and his wife Miriam Reif (member of a Swiss laboratory) (note: it is the Med Cell, the Swiss stem cell factory) a liquid solution based on exosomes to be sprayed was sent in proof [ and, for it, free of any charge, and only to evaluate the effectiveness of its topical use , and all of this on the suggestion of a plastic surgeon (note: it's me!) who had the idea ] to be distributed WITH A SYRINGE without a needle, directly on the wound (the source from adipose tissue counterpart is on deposit at the Med cell), all for 10 days with about 10ml of solution each application, once a day.
Treatment began in late December 2014.
After three applications the regrowth of tissue and narrowing of the plague could be clearly seen.
After eight applications, the wound had reduced by 80% , and within a few days after the 10th and final application the wound was COMPLETELY GONE, raising the total amazement of all operators, to which I never said what the liquid sprayed on the wound was made of.
note: There is no possibility to establish how many exosomes were there in the vial of 10 ml solution, but they have been tested as of human origin: the production process, at Med Cell factory, follows registered steps from a procedure established by an English registered copyright.
Description of the medication technique performed at home with the solution of exosomes, and of the dressing.
The female patient's husband has authorized me to release the data that I expose
The report was written by the female patient's husband himself and is only minimally "adapted" by me.
The treatment deals with a whole of ten procedures of local (topical) application of 10 ml (once a day) of a liquid containing exosomes applied through sprinkling the solution at the time of the daily changing of dressing.
From the sealed vial sent by Med Cell and carefully kept in fridge, a total of 10 ml of solution is taken and applied (after having cleaned with water and soap half an hour before, and then cleaned with sterile saline solution) directly on the wound, inside and outside the area of the bedsore, through a sterile syringe without a needle
The solution is left in contact with the bedsore area for five minutes (on open air) and then a dressing is made through a no sticking material (Allevyn).
N.B. During the treatment the patient has always kept the lying position in bed or on a wheelchair, and never she has been in other positions (for example lying on one or the other side per time).
[Editor's note: EXCURSUS. The application of liquid containing exosomes was performed through "sprayng" or better "sprinkling" with a syringe without a needle by the husband of the patient !!! ... and that topical local application was the new idea! ... But if the patient had been in a protected environment , as in a hospital, I would also suggest inside and at the perimeter microinjections ... but so ... so we had an additional and specific results for a real "topical application" !!]
There is no possibility to establish how many exosomes were there in the vial of 10 ml solution, but they have been tested as of human origin: the production process, at Med Cell factory, follows registered steps from a procedure established by an English registered copyright.
Exosomes and friends.
I forward my experience of many years in fat harvesting and fat transplant (lipografting) for reconstructive purposes and regenerative medicine (fat derived mesenchimal stem cells): I have had extraordinary results in bedsores and in tissue loss after trauma, but also in improving and stimulating recovery after surgery procedures of different type.
__________
Da PubMed e Wikipedia
Cells release into the extracellular environment diverse types of membrane vesicles of endosomal and plasma membrane origin called exosomes and microvesicles, respectively. These extracellular vesicles (EVs) represent an important mode of intercellular communication by serving as vehicles for transfer between cells of membrane and cytosolic proteins, lipids, and RNA.
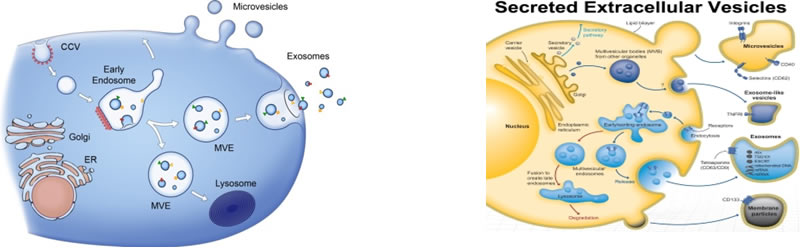
Circulating microvesicles (cMVs) are small membrane bound fragments of between 50 and 1,000 nanometers (nm) in diameter, found in many types of body fluids as well as the interstitial space between cells. Though initially dismissed as cellular debris, cMVs have a role in cell signaling and the process of molecular communication between cells, and are released by a number of cell types. Although a consistent and precise definition is lacking, cMVs are generally considered to be a heterogeneous population of exosomes (<100nm) and shed microvesicles (100-1000nm), which are similar but have distinct mechanisms of formation. Through these mechanisms, cMVs are released into the extracellular space and interact with specific target cells, delivering bioactive molecules.
They have relevance in diseases as cancer, vascular diseases, inflammation, neurological disorders and have clinical application in detection of cancer and as biological markers for disease and in the mechanism of drug delivery
_____________________________
Exosomes mediated resolution of a bedsore
I wish to present a condition of skin ulcer (a bedsore) resolved through the use of local application of exosomes. I think this is the first documented clinical case
It is not a matter of "magic" because the situation of complete recover is still present. Pictures and documentation are added.
It is a single "case report" and no clinical trial has been planned or registered: I happened to find myself in the right condition and in the right place to experiment what is presented.
I was interested in the project of using exosomes in tissue loss treatment, having (because of my work on fat grafting in yr 2000 with Prof Luigi Donati of the University of Milan) had experience on the treatment of loss of substance through transplanted fat, and thinking exosomes could get better results in the loss of substance than fat itself, or maybe after a fat transplant alone and the use of exosomes instead of subsequent additional transplants as it is done now, for example in cases of loss of substance for reconstruction after trauma or cancer or for malformations (Romberg syndrome), or after a reconstruction with a flap in order to improve its viability, or in case of bedsores or, yet better, in severe burns in which (think the importance of what !!) there is no more tissue for reconstruction.
In August 2014, the Med Cell, a Swiss-based in Munchwilen Stem Cell Factory, presented a research project entitled "In vitro studies regarding the production of exosomes and paracrine substances" with the objectives of (1) improving the achievement of exosomes and paracrine substances; (2) create treatment more specifically suited for neurological cardiac muscle and inflammatory conditions; (3) develop pre-clinical basis.
It forwarded a clinical trial program "Present and future uses of the secretome of adipose derived mesenchymal stem cells on skin diseases" in December 2014 for the assessment of the effects of exosomes (derived from stem cells in adipose tissue) that I thought immediately of great interest in Plastic Surgery.
______________
Evaluated the present literature and considered that yet there have been protocols in action, and that there have been no "genetic" contraindications nor any drawbacks owing to the absence of DNA in these structures (exosomes) and without they being "stem cells" and therefore without any risk of them "going mad" and deviating by the standard, and also considering that abroad other doctors have been treating more than three hundreds patients in collaboration with Med Cell without side effects (making it well known the beneficial effect on diseases as rheumatic arthritis through exosomes) , and also considering that there are no guidelines, I had the idea of having a small cohort of patients (ie to have a small number of patients) to be presented to the scientific community after being appointed Scientific Director of a non-profit foundation based in Switzerland (where I even filed my medical license and specialist license in Plastic Surgery) to give an input stimulus to further research... of course with the condition that Patients and family members were all perfectly informed on all the procedure .... to have a set up, for the beginning, on different clinical situations as loss of substance after injuries or in case of skin ulcers, to demonstrate what other colleagues have already achieved in arthritic degenerative diseases.
It 's very important to remember that I could personally realize that a topical application on the skin (with exosomes provided by Med Cell), practically the usual solution applied by "sprinkling" from a syringe without a needle, used on a female patient immobilized for ALS, with decubitus ulcer (bedsore) in the sacral region, had drastically and quickly (within 10 days of application, once a day! and made by family members at home!) healed his bedsore (there are pictures), which lasted eight months with no mention of improvement despite the usual treatments of debridment and care.
So, since considering this topic of my responsibility, even if only a single case, I thought to offer it as a "case report" to the scientific world, even thinking about the possibility of treatment in case of burns with severe loss of tissue, where the use of a material like "exosomes" (!!!) would be the most appropriate (think the importance of what !! ...when there is no tissue for reconstruction).
_____________
Unfortunately the Foundation's work has been suspended for reasons that are only due to very poor chaps feeded with jealousy for business and with commercial interests (think about "how and what" could be the cut down in costs for therapy and managing of these situations !!!) .
Therefore, with the desire to refrain from any commercial interest that, as a gentleman, I do not compete,... I wish to stress, however, that I am interested in claiming the right of having had this idea of using exosomes on tissue regeneration, getting an accelerated healing that is still maintained (and I think this could be the first "case report" documented throughout the procedure, in the world) because I think I could be the first to be entitled to have observed a clinical case in which there could be no misunderstanding on how the result has been achieved nor this clinical case could have been "contaminated" with external therapies (and in fact the previous treatment lasted eight months without any improvement).
I personally think that, for a higher ideal and with no-profit claim, a doctor and a surgeon should have the courage to overcome some limitations for the welfare of patients and the scientific progress.
Returning to the facts, I must stress that my work was done with the characters of formality and honesty that are inherent to the medical profession and in particular to the delicacy of my specialization in Plastic and Reconstructive Surgery.
My practice has always been carried out in respect of medical practice.
According to the Law, no therapy has been carried out in my medical office.
(Available: documentation of report made to the Office of Health in Ticino on the procedure; the brochure of Stem Cell Factory "Med Cell" and of the Clinic in Zurich; the contracts of Stem Cell Factory with patients; agreements with Hospitals).
__________


